Submitting drug, biologic, and medical device documents can be daunting. Our experts compile and publish your submission dossiers, ensuring they are correctly formatted and meet regional regulatory requirements. We handle everything from planning to submission delivery to health authorities.
Keeping your product compliant throughout its lifecycle is crucial. We update, amend, and renew your dossiers to stay current and compliant. Our proactive approach helps you address regulatory changes efficiently.
We use our industry knowledge to develop strategies that simplify the regulatory process. Our consulting services help you understand regulatory pathways, identify potential issues, and optimize your approach for quicker approvals.
Expand your reach globally with our submission services. We manage all submission formats, including eCTD, across regions like the U.S. (FDA), E.U. (EMA), Japan (PMDA), and China (NMPA). We support all your global regulatory activities.
Our quality control processes ensure your submissions are error-free and meet all guidelines. We minimize rejection risks by making sure your dossiers comply with current regulations.
We help you streamline your dossier execution process, transition to eCTD, and set up data visualization solutions for regulatory operations in emerging markets.
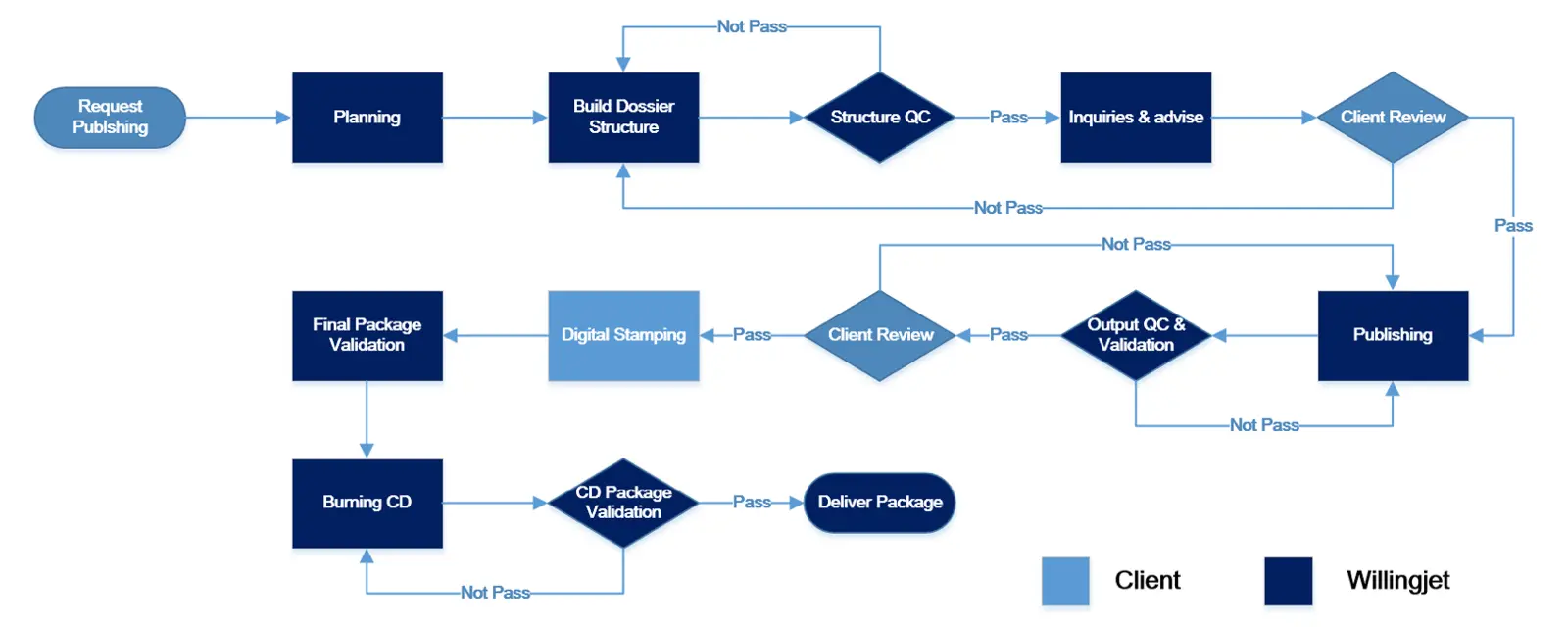
The typical process of our Regulatory Operation service is illustrated in the image below. We offer flexibility in working with either the client's system or our own. Here’s an example of how we handle eCTD submissions:
A leading pharmaceutical company needed help preparing and submitting their New Drug Application (NDA) for a new cancer treatment. They recognized that flawless regulatory submissions were crucial for speeding up the approval process.
The company needed to compile and format a large amount of complex clinical and nonclinical data into a clear submission package. They also had to ensure compliance with stringent requirements across regions like the FDA & EMA.
By partnering with Willingjet, the company accessed tailored regulatory operation services. We worked closely with them to develop a strategic submission plan, leveraging our expertise in oncology and regulatory affairs.
1. Comprehensive Document Management: Our team meticulously organized and compiled all necessary documentation, including clinical trial data, nonclinical studies, and CMC information, into a structured Regulatory Operation format.
2. Regulatory Strategy Development: We worked closely with the client to develop a robust regulatory strategy, identifying potential challenges and optimizing the submission timeline for maximum efficiency.
3. Global Submission Management: Leveraging our expertise in global submissions, we prepared and managed Regulatory Operation submissions for regulatory bodies worldwide, including the FDA and EMA, ensuring compliance with regional requirements.
4. Quality Assurance and Error Correction: We conducted quality checks at every stage to identify and fix errors, providing full support for managing questions from regulatory agencies.
Accelerated Approval Timeline: Through our streamlined Regulatory Operation services, the client successfully navigated the regulatory pathway, accelerating the approval timeline for their groundbreaking cancer therapy.
Compliance Assurance: Our comprehensive approach to regulatory compliance ensured that the submission met all regulatory standards and requirements, minimizing the risk of rejection or delays.
Strategic Partnership: The client benefited from a strategic partnership with Willingjet, gaining access to expert guidance, personalized support, and a proven track record of regulatory success.
Partnering with Willingjet helped the pharmaceutical company expedite regulatory approval for their oncology therapy. Our services and expertise streamlined the submission process, ensured compliance, and ultimately brought life-saving treatments to patients faster.
 What is Regulatory Operation?
What is Regulatory Operation?
Regulatory operations involve submitting dossiers to health agencies in a compliant format, like eCTD, NeES, electronic, and paper. We ensure these dossiers meet all requirements and work with colleagues in all departments to do so.
 What is eCTD?
What is eCTD?
The Electronic Common Technical Document (eCTD) is a standard format for submitting health regulatory applications digitally. It's used globally to submit documents to regulatory authorities efficiently.
 Why is eCTD submission required?
Why is eCTD submission required?
eCTD submission ensures a consistent, organized, and efficient review process for regulatory approvals. Many regulatory agencies, including the FDA, EMA, and PMDA, require it to streamline workflows and speed up decision-making.
 What is the status for eCTD implementation?
What is the status for eCTD implementation?
eCTD is required in many established markets like the U.S., E.U., U.K., Australia, and Canada. It’s voluntary in others, including GCC, Switzerland, South Africa, Japan, China, and Thailand.
 How does your service ensure my submission is compliant with regulatory standards?
How does your service ensure my submission is compliant with regulatory standards?
We stay updated with the latest guidelines and use comprehensive checklists and quality assurance processes to review every part of your submission, ensuring all documents meet necessary standards and formats.
 How can you help us with our global submission strategy?
How can you help us with our global submission strategy?
We help you set up a global submission strategy, managing multiple submissions to ensure all timelines are met. We’re familiar with regulatory operations in all regions and can support your global needs.
 What happens if there are errors in the submission?
What happens if there are errors in the submission?
If we or the regulatory authority find errors, we promptly address and correct them. We provide full support for managing questions from agencies and making necessary revisions to ensure a successful approval process.
 How do you handle updates or amendments to existing eCTD submissions?
How do you handle updates or amendments to existing eCTD submissions?
We manage lifecycle management for your eCTD submissions, including updates, amendments, and renewals, ensuring your dossier is always current and compliant.
 What makes your eCTD services different from others?
What makes your eCTD services different from others?
Our eCTD services stand out due to our expertise, precision, and commitment to client success. We offer personalized attention, tailored strategies, and extensive regulatory knowledge, ensuring compliance and a strategic advantage.
 Key Components of an eCTD Submission?
Key Components of an eCTD Submission?
Here are key components:
Administrative and Prescribing Information: Covers all the essential details about the submission, including application forms and the comprehensive table of contents.
Module 1: Specific to each region and includes regional administrative information.
Module 2: Summaries of the quality, clinical, and nonclinical data.
Module 3: Quality (pharmaceutical documentation and chemistry, manufacturing, and control information).
Module 4: Nonclinical Study Reports.
Module 5: Clinical Study Reports.
Our services simplify the submission process, reducing complexity and eliminating the stress of managing technical requirements. We optimize document management and submission timelines, helping you focus more on innovation.
Stay ahead of regulatory changes with our expert guidance. We ensure your submissions comply with the latest regulations, minimizing rejection risks and speeding up approvals.
Expand your product’s reach with our global submission expertise. We navigate specific regional requirements to facilitate smoother entries into diverse markets, helping you achieve a global footprint.
Speed up your product’s market entry with our efficient submission strategies. We cut down approval times, helping you bring products to market faster.
Gain peace of mind with support from our regulatory affairs experts. We provide personalized assistance throughout the submission process, ensuring guidance every step of the way.
Trust our commitment to data security and integrity. We use advanced security protocols to protect your sensitive information, ensuring confidentiality and security.